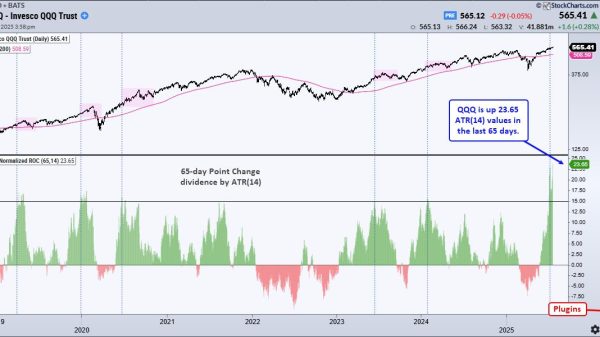
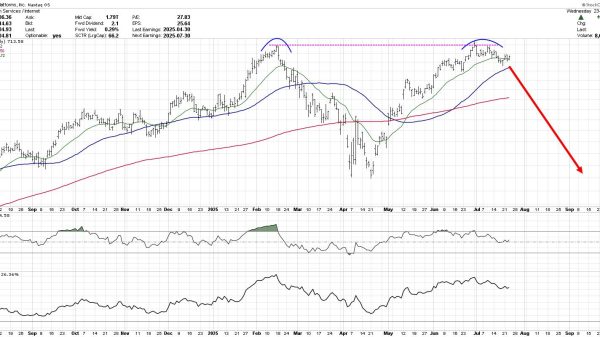
President Donald Trump’s choice to lead the Food and Drug Administration (FDA), Johns Hopkins School of Medicine professor Dr. Marty Makary, cleared a key vote in the Senate on Tuesday, the last such test before his final confirmation vote.
The Senate voted 56-44 to invoke cloture on the nomination.
A final vote to confirm the FDA nominee is slated for after 8 p.m. Tuesday.
Makary, a former Fox News medical contributor, went before the Senate Committee on Health, Education, Labor and Pensions (HELP) earlier this month and answered various questions on vaccines, chronic illness, food safety and abortion.
During his hearing, the nominee faced scrutiny over an FDA vaccine meeting that was reportedly postponed at the last minute.
‘So if you are confirmed, will you commit to immediately reschedule that FDA Vaccine Advisory Committee meeting to get the expert views?’ Sen. Patty Murray, D-Wash., asked Makary at the time.
He responded that he ‘would reevaluate which topics deserve a convening of the advisory committee members on [Vaccines and Related Biological Products Advisory Committee] and which may not require a convening.’
When this response wasn’t good enough for Murray, Makary flipped the question, telling her to confront the Biden administration. ‘Well, you can ask the Biden administration that chose not to convene the committee meeting for the COVID vaccine booster,’ he said.
He was referring to the Biden administration in 2021 pushing through FDA approval for a COVID-19 booster for everyone over the age of 18.
‘The FDA did not hold a meeting of the Vaccines and Related Biological Products Advisory Committee on these actions,’ read a press release at the time, ‘as the agency previously convened the committee for extensive discussions regarding the use of booster doses of COVID-19 vaccines and, after review of both Pfizer’s and Moderna’s EUA requests, the FDA concluded that the requests do not raise questions that would benefit from additional discussion by committee members.’
Committee member Dr. Paul Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, criticized the administration’s move, saying, ‘We’re being asked to approve this as a three-dose vaccine for people 16 years of age and older, without any clear evidence if the third dose for a younger person when compared to an elderly person is of value.’
Makary has long been a critic of the administration he is poised to lead. He wrote an opinion piece in 2021, calling for ‘fresh leadership at the FDA to change the culture at the agency and promote scientific advancement, not hinder it.’
‘We now have a generational opportunity in American healthcare,’ he said at his hearing. ‘President Trump and Secretary Kennedy’s focus on healthy foods has galvanized a grassroots movement in America. Childhood obesity is not a willpower problem, and the rise of early-onset Alzheimer’s is not a genetic cause. We should be, and we will, be addressing food as it impacts our health.’